Our research efforts primarily involve hydrothermal carbonization (HTC) and deep eutectic solvents (DES). We perform HTC of various wet feedstocks including but not limited to municipal solid wastes (MSW), sewage sludge, plastics, waste coal, agricultural residues, and dairy manure. Our goal is develop economically viable and environmentally sustainable HTC processes, which covert aforementioned wet feedstocks to solid fuel, liquid fuel, renewable natural gas, fertilizer, materials for energy storage, and functionalized carbon materials. DES research is targeted to synthesize hydrophobic DES and apply them to recover platform chemicals and organic contaminants from industrial process water.
Please contact Dr. Reza to learn more about the on-going research projects.
SolvX: A Sustainable Chemical Recycling Process for Waste Plastics
End-of-life plastics are an enormous liability for the U.S as well as for the whole world. The U.S. produces more than 35 million tons of plastic per year, but only recycles 2% into similar value-products. The remaining plastic is recycled to lower-value products (7%), incinerated for energy (16%), or landfilled (75%). Plastic bottles composed of polyethylene terephthalate (PET, #01) and high-density polyethylene (HDPE, #02) are the major types of plastics that have been recycled nationally. Whereas most other plastics (#03 - #07) are not recycled or upcycled due to high energy costs and poor economics. Therefore, it is imperative to implement new and sustainable depolymerization technologies to recycle and upcycle end-of-the-life plastics.
Reza Research Group of Florida Institute of Technology (FIT) is proposing solvothermal conversion (SolvX) to chemically recycle waste plastics into platform chemicals and monomers. In this treatment process, waste plastics are treated with organic nonpolar solvents such as toluene, xylene, and benzene at their supercritical conditions. At high temperature, high pressure, and in the presence of catalysts, plastic polymers are broken into monomers. In contrast to mechanical recycling, SolvX might be the ability to produce pure plastic monomers that can be used to produce “virgin” plastics. This will significantly reduce petroleum consumption, energy costs, and carbon emissions compared to traditional PS synthesis. Moreover, SolvX will have advantages over other thermochemical processes, like hydrothermal liquefaction (HTL) and pyrolysis that lose carbon to aqueous and gaseous phases, respectively. In contrast, SolvX uses hydrocarbon solvents at sub- and supercritical conditions to depolymerize plastics without significant carbon loss. Additionally, SolvX operates at lower pressure than HTL and lower temperature than pyrolysis as well as facilitates product separation and solvent recovery via fractional condensation.
Superactivated Hydrochar for Hydrogen Storage
With the global transition towards a sustainable hydrogen (H2) economy, the potential of H2 is explored due to its competent aspect of being a renewable and zero-emission. In addition, H2 has high energy content compared to other alternatives on a gravimetric basis. However, the main challenge of its application is storing in solid-state material which often requires high pressure (around 700 bar) and low temperature (around -196 °C). As a result, the use of H2 energy is still struggling to thrive. Various efforts have been undertaken by storing H2 both chemically and physically. Physical storage has proven more economically viable while considering the recovery of H2. Along with the adsorption parameters (e.g., high microporous surface area, large pore volume, etc.), the adsorption sites and the interaction between adsorption sites with H2 are vital for storing a large volume of H2. Therefore, a material with higher microporosity with active sites will be required to increase the storage of H2.
In the last few years, researchers have reported that the surface area and pore types of materials are the key factors for storing H2. Recently, it has been observed that the presence of oxygen functional groups also plays an important role in uptake H2. However, to the best of authors’ knowledge, no study has been done on the stability of storage materials as they are experienced with low temperature and high pressure. Therefore, the objective of this study was to investigate the structural stability of the H2 storage materials. To achieve this objective, a set of biomass-derived superactivated hydrochars were used in this study. Superactivated hydrochars were prepared from cellulose acetate by hydrothermal carbonization at 220 and 260 °C following by KOH activation at 700 °C for 1 h. The volumetric H2 storage experiments were carried out in High-Pressure Volumetric Analyzer (HPVA) at 100 bar and -196 °C. A total of 9 consecutive cycles of operation were conducted for each superactivated hydrochar. The BET surface area, pore volume, pore size, and volumetric H2 uptake of the material were determined after each cycle. In addition to that, surface morphology of the superactivated hydrochars were evaluated by Scanning Electron Microscope (SEM) analyzer. Analyses showed that the superactivated hydrochars in this study underwent only a 4.5% decrease in the specific surface area and around 1.7% decrease in pore volume after 9 cycles of consecutive H2 adsorption and desorption, signifying high stability of the material for cyclic H2 storage.
Controlling Pyrodinium Outbreaks in the Indian River Lagoon Estuarine System (IRLES) using Low-cost Biochars
The overall objective of this two-year project is to control IRLES’s HABs on low-cost biochars. A team of Chemical and Environmental Engineering, and Marine Sciences experts from Florida Institute of Technology (FIT), University of Central Florida (UCF), and National Oceanographic and Atmospheric Administrations (NOAA) in collaboration with Indian Riven Lagoon National Estuarine Program (IRLNEP), local businesses (Treasure Coast Shellfish, LLC. Green Carbon Solutions, LLC. and Air Burners, Inc.), and local Florida Sea Grant Extension Offices (Brevard, Martin, and St. Lucie Counties) will conduct this FL Sea Grant project by utilizing commercial biochars from waste biomass to control Pyrodinium and STX. The PI will leverage existing facilities to characterize biochars obtained from Air Burners, Inc. and Green Carbon Solutions, LLC. from his federal funding from U.S. Department of Agriculture (USDA, award no: 2019-67019-2928) and National Science Foundation (award number: 1856058). Commercial biochars are prepared from the waste woods of Florida. There are more than 700 million tons of waste wood available in Florida that is causing serious environmental issues including forest fires. Air Burners, Inc. in collaboration with U.S. Forest Services (USFS) is converting this waste wood into biochar with high surface area at a low cost (<$100 per cubic yard). Air Burners, Inc. has several biochar producing equipment with variable throughput up to 100 tons per day. Meanwhile, Green Carbon Solutions (GCS) uses homogeneous wood feedstock in a continuous pyrolysis process that runs 24 hours a day to produce its biochar. Initial capacity of GCS is 5,000 tons of biochar per year with the plan to increase this to 80,000-100,000 tons over the next two plus years. Depending on market demand GCH intends to increase the capacity even further. In this project, various commercial biochars will be collected from Air Burners Inc. and GCH and will be used for Pyrodinium and STX adsorption. We will collaborate with NOAA (letter attached) for Pyrodinium culture and their adsorption on biochar. End user (Treasure Coast Shellfish, LLC,) and regulatory agency (IRLNEP) in collaboration with Florida Sea Grant Extension Offices of Brevard, Martin, St. Lucie Counties will be guiding the project into successful completion.
Prevention and Control of Harmful Algal Bloom
This project is a collaboration among Florida Institute of Technology (FIT), Ohio University (OHIO), and University at Buffalo (UB). The overarching goal is to develop biomass-derived biosorbents capable of controlling and preventing eutrophication. The goal will be accomplished via a combination of systematic experiments at batch-scale to continuous column-scale along with machine learning methods and molecular simulations.
In this project, biosorbents will be synthesized by hydrothermal carbonization (HTC) and thermal activation of model compound (cellulose) and real biomass (corn stover) using recycled HTC process liquid. Adsorption isotherms will be generated for nutrients and microcystin, and will be modeled using machine learning methods to enable prediction of reaction kinetics. Molecular simulations will be performed to study the adsorption of nutrients and microcystin. Model biofilms will be formed and characterized and their effect on adsorption will be studied using continuous flow through experiments and mechanistic modeling. Additionally, work-life of the biosorbent will be evaluated by applying thermal regeneration. Finally, a technoeconomic assessment (TEA) will be performed to determine the economic viability of this technology.
By the end of this project, we will have better understanding of (a) how to synthesize biosorbents for microcystin adsorption, (b) the mechanisms underlying microcystin biosorption, (c) the effects of naturally occurring biofilms on adsorption behavior in a continuous operation, and (d) the process economics involved in biosorbents synthesis and application.
Advanced Biorefinery Feedstock Preparation from Waste Biomass
This project is leveraging Idaho National Laboratory’s (INL) facilities of biomass preprocessing technologies. The overarching goal is to upgrade high-ash biomass into low-ash, mass and energy dense, and pelletized biorefinery feedstocks. The goal will be accomplished via systematic experiments at semi-batch scale to continuous scale and a detailed technoeconomic analysis of a full-scale unit.
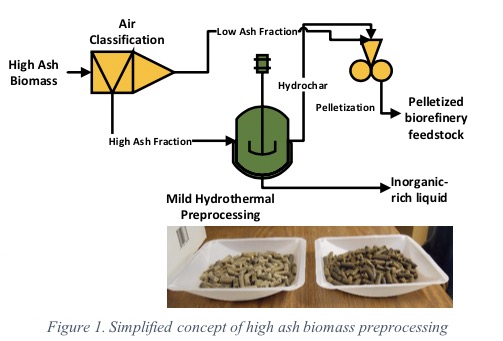 High-ash biomass will be collected from INL’s proven air classification (AC) technology. Mild hydrothermal preprocessing (MHP) will be performed on this high-ash fraction (HAF) biomass reject from AC to reduce structural inorganics. MHP uses subcritical water, which reacts with biomass and leaches inorganics due to its unique thermodynamic properties. Hydrochar, the resulted low ash biomass from MHP, also has binding ability, which will be explored to pelletize low-ash fraction of AC technology in single-press pellet press (at Florida Tech) and pellet mill (at INL). Finally, a detailed technoeconomic analysis of integrated AC, MHP, and pelletization processes will be performed with INL’s guidance.
High-ash biomass will be collected from INL’s proven air classification (AC) technology. Mild hydrothermal preprocessing (MHP) will be performed on this high-ash fraction (HAF) biomass reject from AC to reduce structural inorganics. MHP uses subcritical water, which reacts with biomass and leaches inorganics due to its unique thermodynamic properties. Hydrochar, the resulted low ash biomass from MHP, also has binding ability, which will be explored to pelletize low-ash fraction of AC technology in single-press pellet press (at Florida Tech) and pellet mill (at INL). Finally, a detailed technoeconomic analysis of integrated AC, MHP, and pelletization processes will be performed with INL’s guidance.
By the end of this project, we will have a better understanding of (a) how to remove structural inorganics using MHP technology, (b) the pelletization properties of hydrochar and optimum condition for biomass palletization using MHP hydrochar, and (c) economic feasibility of the high-ash biomass preprocessing technologies. MHP is a unique preprocessing technology that has transformative potentials to improve biorefinery feedstock logistics economics. USDA-NIFA-AFRI has funded $1 M for four years to develop MHP process.
Nexus of Food-Water-Energy in Municipal Solid Waste
Societal challenges around Food, Energy and Water (FEW) systems include in part the overexploitation of resources and increasing resource degradation due to the impacts of current waste stream management. This collaborative project between Florida Tech and Ohio University focuses on systems-level interactions in the framework of "Organic Waste Lifecycles at the Interface of Food, Energy and Water Systems (OWL-FEWs)." The proposed research develops a systems-level framework for the comparative analysis of OWL-FEWs that directly couples behavior and social sciences to material flows and the development of co-products from organic wastes. The project integrates cyber infrastructure to enable real-time analysis of human behavior within waste collection systems, develops a multiscale lifecycle framework for comparing different systems, creates and tests new smart trash bin design (Internet of Things (IoT)), creates organic waste refuse content and tracking technologies, develops an environmentally-friendly deep eutectic solvent to capture carbon dioxide from biogas, and develops new hydrochar processing techniques for the conversion of wet waste as a medium to produce designer hydrochar for water remediation of acid mine drainage, a legacy waste product from coal production.
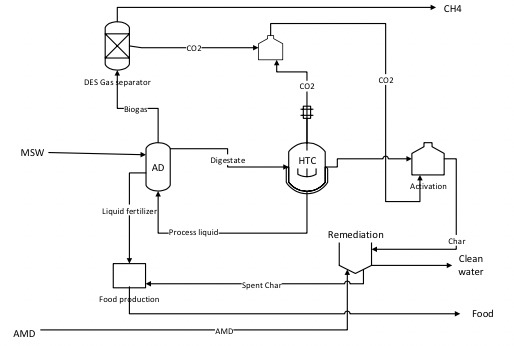
Figure: Nexus of Food Energy Water of food waste
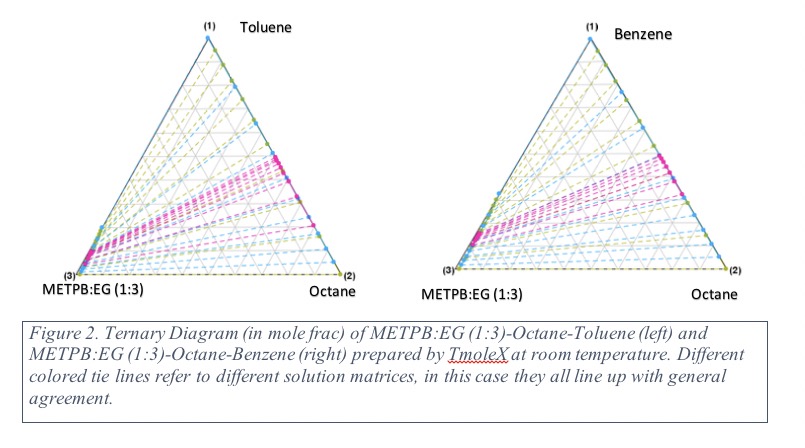
Separation of BTX from Naphtha by DES
This project aims to study thermodynamics and adsorption kinetics of benzene, toluene, and xylene (BTX) into phosphonium-based deep eutectic solvents (DES). Separating BTX from alkanes are challenging as both aromatic and alkane compounds have similar boiling points. In our preliminary studies, methyltriphenyl phosphonium bromide (METPB)-based DES showed high selectivity for BTX due to their available π- electrons in the phenyl orbitals. Until now, most of the DES studies have been performed on equilibrium, although kinetics and thermodynamics studies are necessary to address these following fundamental questions:
- How does DES’s dipolarizability (KT-π* parameter) shift the ternary LLE distribution of BTX/alkane/DES?
- How does the chemical potential of BTX vary with eutectic compositions of DES?
- What is the rate limiting mass transfer step for BTX transfer across two-films?
This project seeks to answer these questions using three specific tasks. First, Kamlet-Taft (KT) parameters (i.e., H-bond donating ability, H-bond accepting ability, and dipolarizability of BTX into DES will be evaluated using solvatochromic method and COnductor like Screening MOdel (COSMO) simulation. Second, partition coefficients for DES as well as sulfolane-assisted BTX separations will be determined from these KT-parameters and ΔS, ΔH, and ΔG will be calculated. Third, mass transfer coefficients of BTX into DES will be estimated using a Lewis cell setup, and self-diffusivity of solute will be measured experimentally and verified by COSMO. By completing this project, we will understand the kinetics of BTX adsorption, which will allow us to design an effective DES process and better utilize its unique properties.

 Give to Florida Tech
Give to Florida Tech 