Research Projects
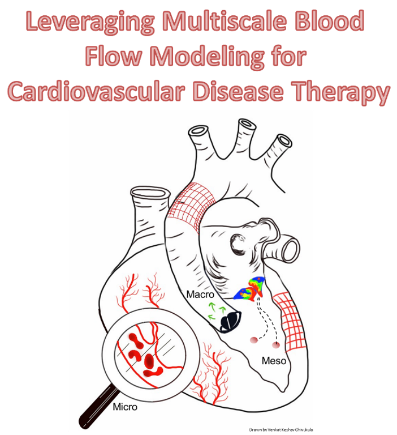
Our primary research area is cardiovascular biomechanics with a focus on multiscale blood flow modeling. Leveraging my strong interdisciplinary computational expertise with complementary in vitro investigations, I seek to develop hemodynamics-driven, evidence-based mechanistic methodologies to understand, detect, diagnose and treat cardiovascular disease. I envision a future where personalized medicine will deliver on its promise and I plan to contribute towards it by incorporating synergistic and interdisciplinary technologies that improve patient outcomes.
In the MCFL, we focus on understanding blood flow (hemodynamics) under healthy and pathological conditions to understand, detect, diagnose and treat cardiovascular disease. We collaborate very closely with clinicians (cardiology, cardiac surgery, vascular surgery, neurosurgery) to ensure our research is always translational, i.e. patient-focused.
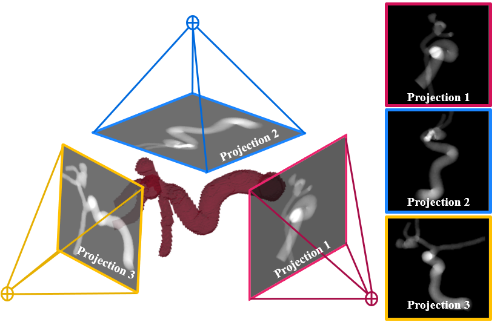
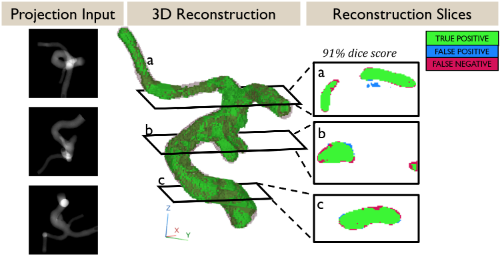
We use a combination of tools such as computational fluid dynamics (CFD) modeling of blood flow, virtual surgery and optimization, reduced order modeling of the cardiovascular system, predictive modeling, 3D printing and rapid prototyping.
 Some of the current / previous projects in the lab focus on:
Some of the current / previous projects in the lab focus on:
- Heart failure therapy
- Life-support devices (Extracorporeal Membrane Oxygenation or ECMO)
- Cerebral aneurysms
- Abdominal aortic aneurysms
- Platelet activation / thrombosis
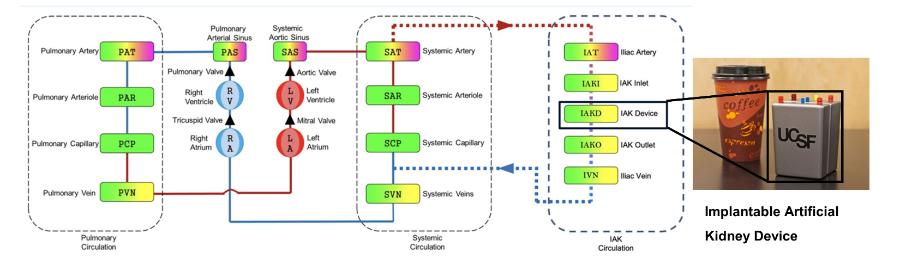
- Medical devices / implants
- Congenital heart diseases
- Virtual surgery and patient management
- Blood particulate interactions
- 3D printing and rapid prototyping of cardiovascular blood vessel models
Looking for graduate / undergraduate researchers!
Potential projects include (but not limited to): heart failure, cardiogenic shock, stroke, atherosclerosis, atrial fibrillation, 3D computational fluid dynamics simulations of blood flow in the heart and various blood vessels, reduced order modeling of the cardiovascular network, Lagrangian tracking of individual red blood cells / platelets, platelet activation modeling, development of computational techniques, machine learning, 3d printing, rapid prototyping.
Preferred background
Curiosity about the cardiovascular system and how medical therapy works, experience with computational analysis software such as MATLAB / Python / C / C++, design software such as Solidworks / Autocad, computational modeling (such as CFD or finite element modeling and meshing), 3D printing, rapid prototyping. Familiarity with fluid mechanics, cardiovascular physiology, cardiovascular pathologies and/or medical therapies is ideal. Inquisitiveness and an open mind are essential!
If you are interested, please email me at vchivukula@fit.edu with your resume and transcripts


-774x681.png)